Background
Although the survival rate of adolescents and young adults (AYA) with acute lymphoblastic leukemia (ALL) has improved by using pediatric-based treatment protocols, those who experience a relapse still have poor outcomes when treated with adult chemotherapy salvage-regimens, which have traditionally included high doses of Ara-C.
The long-term survival rate for patients treated with these salvage-regimens is less than 10%. Salvage treatment with blinatumomab for adults with relapsed B-cell ALL results in a complete remission (CR) rate of 34%, a median survival of 7.7 months, and an overall survival (OS) of less than 30% at 18 months.
In contrast, the ALLR3 protocol for relapsed ALL in patients up to the age of 18 years achieved a CR rate of 97% and an overall survival rate of 72%.
Therefore, this study aims to compare the outcomes of AYA patients treated with pediatric-inspired regimen (R3 regimen) to those treated with adult-based regimen (FA (Fludarabine/Cytarabine) protocol).
Methods
The R3 protocol treated 17 AYA patients with relapsed Philadelphia-negative ALL, aged between 14 to 40 years old, who had received first-line therapy. Patients who achieved CR underwent allogenic transplant. The protocol included vincristine, mitoxantrone, asparaginase, dexamethasone, and intrathecal methotrexate.
In comparison, 60 patients were treated with FA protocol, with patients achieved CR proceeded to allogeneic stem cell transplantation. Relapses were classified as very early (< 18 months from diagnosis), early (>18 months from diagnosis and <6 months from the end of therapy), and late (>6 months from the end of therapy). Patients' characteristics, response rate (RR) and overall survival (OS) were analyzed.
Results
The two arms had comparable characteristics.
The median age in the R3 arm was 16 years (range: 14-40 years) compared to 22 years (range: 18-32 years) in the FA protocol arm. In the R3 arm, 88.2% of patients had B-cell ALL, while in the FA protocol arm, 92.2% had B-cell ALL.
CNS involvement was seen in 28.8% and 17.6% of cases in the R3 and FA protocol arms, respectively.
Relapses were classified as very early, early, or late in 2 (12%), 5 (29%), and 7 (41%) of cases in the R3 arm and were mostly late (25.2%) in the FA protocol arm. Relapses in the R3 arm were isolated bone marrow (BM), isolated extramedullary (EMD), or combined BM and EMD in 14 (82%), 1 (6%), and 2 (12%) cases, respectively, while EMD was around three times higher (16.9%) in the FA arm.
The overall CR rate was 60% at the end of R3 induction, with negative MRD (<10 −4 cells) in 47.1% of patients. The overall CR rate was higher in the FA arm (76.3%), but the difference with the R3 arm was not statistically significant (p-value 0.1). The disease was refractory to subsequent salvage therapy in 42% of cases in both arms. About half of the patients who responded to R3 proceeded to allogeneic stem cell transplant (52.3%), while 78% of the patients post-FA underwent transplantation.
Eight patients died (47%) in R3 arm, but only one patient died due to treatment-related sepsis, while the other seven patients died due to disease-related causes.
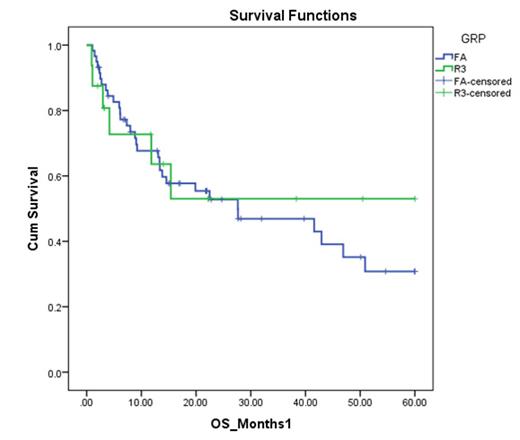
At a median follow-up of 4 years, OS was 53% in the R3 arm compared to 30.8% in the FA arm, but the difference was not significant (p-value=0.8).
The post-FA mortality was mainly due to infections (15.3% in FA vs. 5.9% in R3).
Conclusion
Pediatric-inspired protocols that have shown significant benefits in the front-line setting for ALL in the AYA population did not demonstrate a significant difference when compared to traditional adult-based salvage chemotherapy regimens in relapsed disease.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal